MUSHROOM CONTAMINATION: HOW TO SPOT AND WHAT TO DO
October 22, 2021
In the world of mushroom cultivation, contamination is as inevitable as death and taxes. That’s why a solid understanding and consistent practice of the fundamentals of sterile technique is so important.
Yet even with a flawless workflow, contamination will at some point find its way into your grow bag, grain jar, Petri dish, or monotub. All that’s needed is a nutrified substrate, a weak mycelial culture, and a chance. When that moment arrives, you want to know how to spot mushroom contamination, and just as importantly, what to do about it.

Mushroom Contamination #1:
Trichoderma
Trichoderma is a genus of green mold that preys on other fungal mycelium and is easily distinguished by its vibrant blue-green color during sporulation. Of all the types of contamination you may encounter, trichoderma is one of the easiest to identify—especially after sporulation—and one of the most difficult to contain. Trichoderma harzianum is the most common and is known to produce an aggressive white mycelium that causes a soft decay in mushrooms before it sporulates into a vibrant green.
How to Spot
Trying to identify and eradicate trichoderma when it is in the stage of growth right before sporulation is difficult yet vital to save your crop. At this point in its life cycle, it can appear similar to mycelium. In the photo below, note the thick, fluffy appearance on trichoderma rising from the substrate. Mycelium does not look like this. Instead, mycelium appears more rope-like and stays tight to the substrate.
Once trichoderma sporulates, though, it’s practically impossible to misidentify. In the photo below, note the green splotches characteristic of trichoderma.

What to Do
Trichoderma is notorious for being practically impossible to contain once it takes hold. If it starts to sporulate, your best bet is to get the trichoderma-contaminated petri dish, grain jar, grow bag, or monotub as far away from your lab and/or fruiting room as quickly as possible. If you don’t, you risk it spreading and your mushroom contamination issue turning into a full-fledged crisis as it contaminates neighboring dishes, jars, bags, and tubs.
Nonetheless, if you’re hell bent on trying to save whatever it is that’s contaminated, spreading salt on the affected area is one approach used in Agaricus mushroom production farms when encountering trichoderma. We don’t recommend this approach unless it’s, say, your only tub. We think simply cutting your losses and ditching the trich’ train altogether is a better idea.
After you spot, identify, and rid yourself of trichoderma, it is a good idea to sanitize everything in the affected area as much as possible before beginning another grow. It’s also smart to look back at your processes to attempt at identifying where the mushroom contamination issue likely began (more on that later).
Mushroom Contamination #2:
Bacteria, e.g. Bacillus spp.
When it comes to bacterial mushroom contamination, the most common type is Bacillus spp., also known as “wet spot” or “sour rot.” Mushroom cultivators typically soak their grains for 12 to 24 hours before hydrating or sterilizing them.
Why?
Because bacterial endospores can be heat resistant, meaning they’ll survive the pressure-cooking process. By soaking the grains overnight, we circumvent this issue. In essence, soaking the grains causes any bacterial endospores on the grains to awake and germinate. Then, once the grains reach sterilization temperatures during the pressure-cooking process, those pesky heat resistant endospores die off. Without the soak, the endospores could lay dormant and survive the sterilization process, coming to life after you’ve already inoculated your grains and ruining your grow.

How to Spot
In grains, bacterial contamination appears dull gray, slimy, excessively wet, similar in appearance to mucus, and is easy to identify by taking a big ‘ole whiff of your grain jar when you suspect it is present. As the nickname “sour rot” suggests, if your grains smell sour, you’re probably dealing with Bacillus spp.
As for its appearance on petri dishes, bacterial contamination looks like a slimy, wet patch, hence the nickname “wet spot.” In the photo below, bacterial contamination is identified as the wet looking slime stretching out in finger-like formations from the patch of white mycelium.
As for its appearance on petri dishes, bacterial contamination looks like a slimy, wet patch, hence the nickname “wet spot.” In the photo below, bacterial contamination is identified as the wet looking slime stretching out in finger-like formations from the patch of white mycelium.
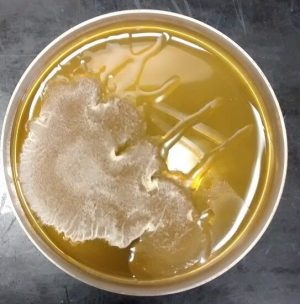
What to Do
The best way to avoid bacterial mushroom contamination like Bacillus spp. is to soak your grains for 12 to 24 hours at room temperature before you begin the sterilization process. In this way, the heat resistant endospores, if viable, will germinate and then be susceptible to the standard sterilization process.
If you encounter bacteria on a petri dish, it can be a fun experiment to let it sit for a few days to see how your mycelium culture responds. Perhaps it will consume the bacteria. If it does, voila, you have a wicked strong culture capable of fighting off bacteria. Bacterium do sporulate but not to the degree of trichoderma so the risk of it spreading to other plates isn’t as high. Nonetheless, it’s always a good idea to separate your clean cultures from the contaminated ones.
If bacterial mushroom contamination is encountered on grain, it is possible to separate the bacterially contaminated grains from the rest of the healthy myceliated grains. However, this usually only works if the bacterial patch is small and contained to one area. To accomplish this separation, scoop out the bacterial patch using an alcohol sanitized or flame sterilized spoon. This can also be done in a monotub with an isolated patch of bacteria, which often can be identified by a yellowing of the substrate, seen in the photo above. Note that in the photo, the bacteria isn’t yellow. Instead, the yellowing represents the mycelium’s reaction to the bacteria as the mycelium secretes metabolites/enzymes to protect itself.
Mushroom Contamination #3:
Cobweb Mold (Dactylium Mildew), e.g. Hypomyces spp.
Cobweb mold is not one species of mold but rather a closely related group of mold species that tend to cause soft rot in mushrooms. Usually, one encounters cobweb mold in grain spawn and monotubs.

How to Spot

Cobweb mold can be difficult to spot because it appears grey, white, and fluffy, similar to mycelium. Typically, it grows three-dimensionally, levitating above the substrate in wispy, white tufts. Cobweb mold spreads fast, causes your baby mushrooms to abort their growth, and in some cases can prevent your mushrooms from growing at all. When cobweb mold contacts a mushroom, the mold soon envelopes the mushroom with its soft, mildewy mycelium and causes a soft rot in the mushroom. Cobweb is also a parasite of wild mushrooms.
To identify cobweb mold, you must learn how to distinguish it from mycelium. Cobweb mold is usually greyer and more wispy than typical mycelium. It possesses very fine strands when compared to the rope-like hyphal growth of mycelium, and has the smell of mildew. If you open your tub to the sight of wispy gray tufts growing three-dimensionally and the scent of a damp basement meets your nostrils, you’ve likely just encountered cobweb mold. In the photo below, note the clouds of wispy grey growing upward from the substrate. This is cobweb mold. The more vibrant but smaller white patches located near the sides of the container are mycelium.
Another way to identify cobweb mold is by the sheer speed of its growth. A small patch the size of a penny can grow to cover an entire jar or tub in 24 to 48 hours. In the photo below, cobweb mold on grain is distinguished by its light wispy appearance when compared to the more vibrant white patches of mycelium.

What to Do
If spotted early, you can control cobweb mold and salvage your grow using hydrogen peroxide. Hydrogen peroxide (H2O2) spray is a great weapon in the battle against mold because it will not harm your mycelium but will kill any mold and/or spores trying to take over your monotub. So spray, spray, spray away.
To limit the chances of encountering cobweb mold, make sure your grains/substrate aren’t overhydrated since cobweb mold prefers high humidity (i.e. an overhydrated substrate) and stagnant air. By lowering the humidity and providing more air circulation, you can help limit the potential for and spread of cobweb mold mushroom contamination. After a H2O2 spray down, soaking a paper towel in H202 and laying it where the contamination once was is another strategy and can be used for trichoderma and bacterial contamination, too.
Where You Went Wrong
The most common cause of mushroom contamination is incorrect sterilization. If you create a nutrified substrate that’s not completely sterilized, you’re basically placing a fat plate of food in front of a table of hungry hippos. One of those hippos, or maybe a few of them, are going to eat. Your mycelium culture will then go hungry, weaken, and lose out. Poor sterile workflow is another common cause, as is working with weak mycelial cultures or trying to cut corners with your preparation.
As for where exactly the contamination is coming from, you don’t have to look far. In fact, go take a look in the mirror. You, you dirty human, are one heck of a vector for mushroom contamination. There are billions of microorganisms on you right now. Yuck! Take a shower before you do any sterile lab work to limit the chance of those little guys jumping into your grow.
If you practice good sterile technique, make sure you properly prepare and sterilize your substrates, and ensure you’re as clean as you can be before working with sterile substrates, you’ll definitely increase your chance at success. And if you find yourself overjoyed with the process and ever thirsty for more, you may have just found your calling at the perfect time.
You know what else increases your chance at success? Taking our online or attending our physical mushroom cultivation courses, where we cover every step of the process in a hands-on environment alongside people just like you!

October 22, 2021
In the world of mushroom cultivation, contamination is as inevitable as death and taxes. That’s why a solid understanding and consistent practice of the fundamentals of sterile technique is so important.
Yet even with a flawless workflow, contamination will at some point find its way into your grow bag, grain jar, Petri dish, or monotub. All that’s needed is a nutrified substrate, a weak mycelial culture, and a chance. When that moment arrives, you want to know how to spot mushroom contamination, and just as importantly, what to do about it.
Mushroom Contamination #1:
Trichoderma
Trichoderma is a genus of green mold that preys on other fungal mycelium and is easily distinguished by its vibrant blue-green color during sporulation. Of all the types of contamination you may encounter, trichoderma is one of the easiest to identify—especially after sporulation—and one of the most difficult to contain. Trichoderma harzianum is the most common and is known to produce an aggressive white mycelium that causes a soft decay in mushrooms before it sporulates into a vibrant green.
How to Spot
Trying to identify and eradicate trichoderma when it is in the stage of growth right before sporulation is difficult yet vital to save your crop. At this point in its life cycle, it can appear similar to mycelium. In the photo below, note the thick, fluffy appearance on trichoderma rising from the substrate. Mycelium does not look like this. Instead, mycelium appears more rope-like and stays tight to the substrate.
Once trichoderma sporulates, though, it’s practically impossible to misidentify. In the photo below, note the green splotches characteristic of trichoderma.
What to Do
Trichoderma is notorious for being practically impossible to contain once it takes hold. If it starts to sporulate, your best bet is to get the trichoderma-contaminated petri dish, grain jar, grow bag, or monotub as far away from your lab and/or fruiting room as quickly as possible. If you don’t, you risk it spreading and your mushroom contamination issue turning into a full-fledged crisis as it contaminates neighboring dishes, jars, bags, and tubs.
Nonetheless, if you’re hell bent on trying to save whatever it is that’s contaminated, spreading salt on the affected area is one approach used in Agaricus mushroom production farms when encountering trichoderma. We don’t recommend this approach unless it’s, say, your only tub. We think simply cutting your losses and ditching the trich’ train altogether is a better idea.
After you spot, identify, and rid yourself of trichoderma, it is a good idea to sanitize everything in the affected area as much as possible before beginning another grow. It’s also smart to look back at your processes to attempt at identifying where the mushroom contamination issue likely began (more on that later).
Mushroom Contamination #2:
Bacteria, e.g. Bacillus spp.
When it comes to bacterial mushroom contamination, the most common type is Bacillus spp., also known as “wet spot” or “sour rot.” Mushroom cultivators typically soak their grains for 12 to 24 hours before hydrating or sterilizing them.
Why?
Because bacterial endospores can be heat resistant, meaning they’ll survive the pressure-cooking process. By soaking the grains overnight, we circumvent this issue. In essence, soaking the grains causes any bacterial endospores on the grains to awake and germinate. Then, once the grains reach sterilization temperatures during the pressure-cooking process, those pesky heat resistant endospores die off. Without the soak, the endospores could lay dormant and survive the sterilization process, coming to life after you’ve already inoculated your grains and ruining your grow.
How to Spot
In grains, bacterial contamination appears dull gray, slimy, excessively wet, similar in appearance to mucus, and is easy to identify by taking a big ‘ole whiff of your grain jar when you suspect it is present. As the nickname “sour rot” suggests, if your grains smell sour, you’re probably dealing with Bacillus spp.
As for its appearance on petri dishes, bacterial contamination looks like a slimy, wet patch, hence the nickname “wet spot.” In the photo below, bacterial contamination is identified as the wet looking slime stretching out in finger-like formations from the patch of white mycelium.
As for its appearance on petri dishes, bacterial contamination looks like a slimy, wet patch, hence the nickname “wet spot.” In the photo below, bacterial contamination is identified as the wet looking slime stretching out in finger-like formations from the patch of white mycelium.
What to Do
The best way to avoid bacterial mushroom contamination like Bacillus spp. is to soak your grains for 12 to 24 hours at room temperature before you begin the sterilization process. In this way, the heat resistant endospores, if viable, will germinate and then be susceptible to the standard sterilization process.
If you encounter bacteria on a petri dish, it can be a fun experiment to let it sit for a few days to see how your mycelium culture responds. Perhaps it will consume the bacteria. If it does, voila, you have a wicked strong culture capable of fighting off bacteria. Bacterium do sporulate but not to the degree of trichoderma so the risk of it spreading to other plates isn’t as high. Nonetheless, it’s always a good idea to separate your clean cultures from the contaminated ones.
If bacterial mushroom contamination is encountered on grain, it is possible to separate the bacterially contaminated grains from the rest of the healthy myceliated grains. However, this usually only works if the bacterial patch is small and contained to one area. To accomplish this separation, scoop out the bacterial patch using an alcohol sanitized or flame sterilized spoon. This can also be done in a monotub with an isolated patch of bacteria, which often can be identified by a yellowing of the substrate, seen in the photo above. Note that in the photo, the bacteria isn’t yellow. Instead, the yellowing represents the mycelium’s reaction to the bacteria as the mycelium secretes metabolites/enzymes to protect itself.
Mushroom Contamination #3:
Cobweb Mold (Dactylium Mildew), e.g. Hypomyces spp.
Cobweb mold is not one species of mold but rather a closely related group of mold species that tend to cause soft rot in mushrooms. Usually, one encounters cobweb mold in grain spawn and monotubs.
How to Spot
Cobweb mold can be difficult to spot because it appears grey, white, and fluffy, similar to mycelium. Typically, it grows three-dimensionally, levitating above the substrate in wispy, white tufts. Cobweb mold spreads fast, causes your baby mushrooms to abort their growth, and in some cases can prevent your mushrooms from growing at all. When cobweb mold contacts a mushroom, the mold soon envelopes the mushroom with its soft, mildewy mycelium and causes a soft rot in the mushroom. Cobweb is also a parasite of wild mushrooms.
To identify cobweb mold, you must learn how to distinguish it from mycelium. Cobweb mold is usually greyer and more wispy than typical mycelium. It possesses very fine strands when compared to the rope-like hyphal growth of mycelium, and has the smell of mildew. If you open your tub to the sight of wispy gray tufts growing three-dimensionally and the scent of a damp basement meets your nostrils, you’ve likely just encountered cobweb mold. In the photo below, note the clouds of wispy grey growing upward from the substrate. This is cobweb mold. The more vibrant but smaller white patches located near the sides of the container are mycelium.
Another way to identify cobweb mold is by the sheer speed of its growth. A small patch the size of a penny can grow to cover an entire jar or tub in 24 to 48 hours. In the photo below, cobweb mold on grain is distinguished by its light wispy appearance when compared to the more vibrant white patches of mycelium.
What to Do
If spotted early, you can control cobweb mold and salvage your grow using hydrogen peroxide. Hydrogen peroxide (H2O2) spray is a great weapon in the battle against mold because it will not harm your mycelium but will kill any mold and/or spores trying to take over your monotub. So spray, spray, spray away.
To limit the chances of encountering cobweb mold, make sure your grains/substrate aren’t overhydrated since cobweb mold prefers high humidity (i.e. an overhydrated substrate) and stagnant air. By lowering the humidity and providing more air circulation, you can help limit the potential for and spread of cobweb mold mushroom contamination. After a H2O2 spray down, soaking a paper towel in H202 and laying it where the contamination once was is another strategy and can be used for trichoderma and bacterial contamination, too.
Where You Went Wrong
The most common cause of mushroom contamination is incorrect sterilization. If you create a nutrified substrate that’s not completely sterilized, you’re basically placing a fat plate of food in front of a table of hungry hippos. One of those hippos, or maybe a few of them, are going to eat. Your mycelium culture will then go hungry, weaken, and lose out. Poor sterile workflow is another common cause, as is working with weak mycelial cultures or trying to cut corners with your preparation.
As for where exactly the contamination is coming from, you don’t have to look far. In fact, go take a look in the mirror. You, you dirty human, are one heck of a vector for mushroom contamination. There are billions of microorganisms on you right now. Yuck! Take a shower before you do any sterile lab work to limit the chance of those little guys jumping into your grow.
If you practice good sterile technique, make sure you properly prepare and sterilize your substrates, and ensure you’re as clean as you can be before working with sterile substrates, you’ll definitely increase your chance at success. And if you find yourself overjoyed with the process and ever thirsty for more, you may have just found your calling at the perfect time.
You know what else increases your chance at success? Taking our online or attending our physical mushroom cultivation courses, where we cover every step of the process in a hands-on environment alongside people just like you!



